Actively Recruiting long COVID Studies:
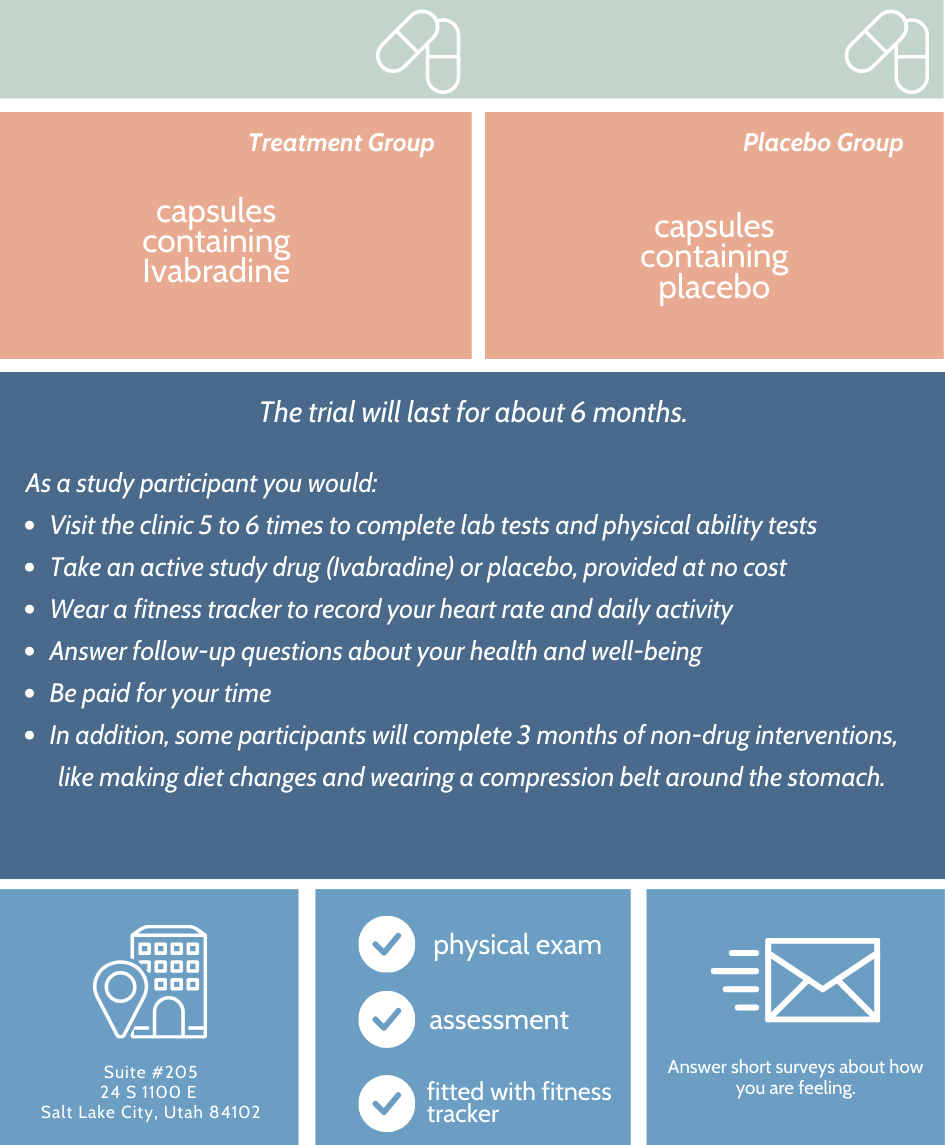
A Randomized Double Blind Placebo Control Trial to Determine the Effects of Ivabradine and lifestyle changes on Long COVID.
What is being studied?
Researchers are studying possible treatments for adults who have Postural Orthostatic Tachycardia Syndrome (POTS) symptoms related to Long COVID. POTS causes a number of autonomic dysfunction symptoms like fast heart rate, dizziness, and fatigue when standing up from sitting or lying down to standing.
What do we hope to learn?
We want to learn if a study drug called Ivabradine and lifestyle changes can improve daily functioning for people with ongoing symptoms after a COVID infection. Click here for the study flyer.
What is the study eligibility?
You may be a good fit for this study if you are an adult who developed POTS symptoms after getting COVID, you do not have an active COVID infection, you still have one or more of these POTS symptoms when you stand up: fast heart rate, dizziness, fatigue.
Interested in participating?
Contact Annaleah Otteson in the Research Department at Bateman Horne Center by calling 801-532-8311 or emailing [email protected]
Participants will have an equal chance of receiving either the active study drug (Ivabradine) or placebo.
Bateman Horne Center conducts high-standard, quality clinical research and trials, assuring the research question is answered in a reliable, valid, and unbiased manner, while protecting the rights and welfare of human subjects.
Bateman Horne Center follows standardized procedures and processes, ensuring regulatory compliance and the safety of human subjects.
Research Publications
The following publications are a result of BHC led and/or partnered research findings
Incidence and Prevalence of Post-COVID-19 Myalgic Encephalomyelitis: A Report from the Observational RECOVER-Adult Study
Description: RECOVER-Adult is a longitudinal observational cohort study conducted across the U.S. We included participants who had a study visit at least 6 months after infection and had no pre-existing ME/CFS, grouped as (1) acute infected, enrolled within 30 days of infection or enrolled as uninfected who became infected (n=4515); (2) post-acute infected, enrolled greater than 30 days after infection (n=7270); and (3) uninfected (1439). View the full publication in Journal of General Internal Medicine.
Cognitive impairment in post-acute sequelae of COVID-19 and short duration myalgic encephalomyelitis patients is mediated by orthostatic hemodynamic changes
Importance: For PASC patients, both their disease state and hemodynamic changes during orthostatic challenge were associated with slower reaction time and decreased response accuracy during cognitive testing. Reduced cognitive efficiency in <4 year ME/CFS patients was associated with higher heart rate in response to orthostatic stress. Hemodynamic changes did not correlate with cognitive impairment for >10 year ME/CFS patients, but cognitive impairment remained. These findings underscore the need for early diagnosis to mitigate direct hemodynamic and other physiological effects on symptoms of cognitive impairment. View the full publication in Frontiers in Neuroscience.
Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
Importance: All but one Long COVID respondent reported having PEM. There were many significant differences in the types of PEM triggers, symptoms experienced during PEM, and ways to recover and prevent PEM between Long COVID and ME/CFS. Similarities between Long COVID and ME/CFS included low and medium physical and cognitive exertion to trigger PEM, symptoms of fatigue, pain, immune reaction, neurologic, orthostatic intolerance, and gastrointestinal symptoms during PEM, rest to recover from PEM, and pacing to prevent PEM.
View the full publication in the journal of WORK.
Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS
Importance: In this study, we employed shotgun metagenomics and metabolomics to assess dysbiosis in the largest prospective case-control study to date, consisting of 106 ME/CFS subjects and 91 matched healthy controls. Our findings provide insights into disturbances in the microbiome and their relationship with fatigue symptoms in ME/CFS.
View the full publication in the journal of Cell Host & Microbe.
Multi-‘omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients
Importance: The etiology of chronic fatigue syndrome (CFS) is largely unknown. In this issue of Cell Host and Microbe, Guo et al. and Xiong et al. report CFS-associated gut microbiome and metabolomic datasets-implicating dysregulation of immune-modulating molecules. This may provide a framework for new therapeutic paradigms and disease origins.
View the full publication in the journal of Cell Host & Microbe.
Improvement of Long COVID symptoms over one year
View the full publication in the journal of Frontiers in Medicine.
Orthostatic Challenge Causes Distinctive Symptomatic, Hemodynamic and Cognitive Responses in Long COVID and ME/CFS
Background: Some patients with acute COVID-19 are left with persistent, debilitating fatigue, cognitive impairment (“brain fog”), orthostatic intolerance (OI) and other symptoms (“Long COVID”). Many of the symptoms are like those of other post-infectious fatigue syndromes and may meet criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Common diagnostic laboratory tests are often unrevealing.
View the full publication in the journal of Frontiers in Medicine: Family and Primary Care
Dissecting the Nature of Post-Exertional Malaise
Background: Post-exertional malaise (PEM) is a defining characteristic of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) but there is insufficient research dissecting the nature of PEM from the patients’ perspective.
View the full publication in the journal of Fatigue : Biomedicine, Health & Behavior.
Accurate and objective determination for ME/CFS disease severity with a wearable sensor
Background: Approximately 2.5 million people in the U.S. suffer from myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). This disease negatively impacts patients’ ability to function, often resulting in difficulty maintaining employment, sustaining financial independence, engaging socially with others, and in particularly severe cases, consistently and adequately performing activities of daily living. The focus of this research was to develop a sensor-based method to measure upright activity defined as time with feet on the floor and referred to as UpTime, as an indicator of ME/CFS disease severity.
View the full publication in the Journal of Translational Medicine.
Hemodynamics during the 10-minute NASA Lean Test: Evidence of circulatory decompensation in a subset of ME/CFS patients
Background: Lightheadedness, fatigue, weakness, heart palpitations, cognitive dysfunction, muscle pain, and exercise intolerance are some of the symptoms of orthostatic intolerance (OI). There is substantial comorbidity of OI in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome). The 10-minute NASA Lean Test (NLT) is a simple, point-of-care method that can aid ME/CFS diagnosis and guide management and treatment of OI. The objective of this study was to understand the hemodynamic changes that occur in ME/CFS patients during the 10-minute NLT.
View the full publication in the Journal of Translational Medicine.
Clinically accessible tools for documenting the impact of orthostatic intolerance on symptoms and function in ME/CFS
Background: Clinical observations have indicated that hours of upright activity (HUA) reported by Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) patients correlated with orthostatic symptoms and impaired physical function. This study examined the relationship between HUA and orthostatic intolerance (OI).
View the full publication in Work.
 Lucinda Bateman, MD, is a renowned clinician, researcher, and educator. Her Johns Hopkins University Medical School training instilled an approach to care that she has employed throughout her career - the patient comes first and the unknown or unexplained does not equate to a lack of proper and compassionate care. Since starting her own practice in 2000, she has served on six boards or committees, been the principal investigator for 45 studies, authored/coauthored 40 journal articles, served as adjunct instructor and adjunct assistant professor in the University of Utah Departments of Preventative Medicine, Internal Medicine, and Anesthesiology, and lectured around the world.
Lucinda Bateman, MD, is a renowned clinician, researcher, and educator. Her Johns Hopkins University Medical School training instilled an approach to care that she has employed throughout her career - the patient comes first and the unknown or unexplained does not equate to a lack of proper and compassionate care. Since starting her own practice in 2000, she has served on six boards or committees, been the principal investigator for 45 studies, authored/coauthored 40 journal articles, served as adjunct instructor and adjunct assistant professor in the University of Utah Departments of Preventative Medicine, Internal Medicine, and Anesthesiology, and lectured around the world.