Dr. Suzanne D. Vernon, BHC research liaison, explains how the IOM report defining the core symptoms of ME/CFS also define the BHC research roadmap.
________

Suzanne D. Vernon, PhD BHC Research Liaison
At the October 2012 CFS Advisory Committee meeting Dr. Sandra Kweder, Deputy Director of the Office of New Drugs at the FDA stated, “When there is confusion, lack of consensus and no progress, go back to the core”. Dr. Kweder was explaining why there are no FDA approved drug treatments for ME/CFS. She told the audience that by “core” she meant the disease-defining concepts that describe the signs, symptoms and decrements in specific functioning experienced by patients. Dr. Kweder went further to say that without these core-defining concepts investment in developing treatments for ME/CFS would be slow. She asked, “Would you invest in developing a treatment surrounded by such uncertainty?”
But the FDA was intent on moving the regulatory path forward for ME/CFS and in March 2014 the FDA published draft industry guidance for developing drug products for ME/CFS treatment. Because ME/CFS disease defining concepts were still uncertain, this guidance indicated that, “At this time, the FDA does not recognize any particular disease definition, nomenclature, or diagnostic criteria for CFS/ME as the most appropriate for use in clinical trials of new drug products” leaving the door open for sponsors to define ME/CFS (with adequate justification) and target subpopulations like orthostatic intolerance, disturbed sleep, etc. in their clinical trials. The guidance recommended that drug efficacy be determined by patient-reported outcomes – how a patient feels or functions – and that biomarkers could be used as exploratory endpoints.
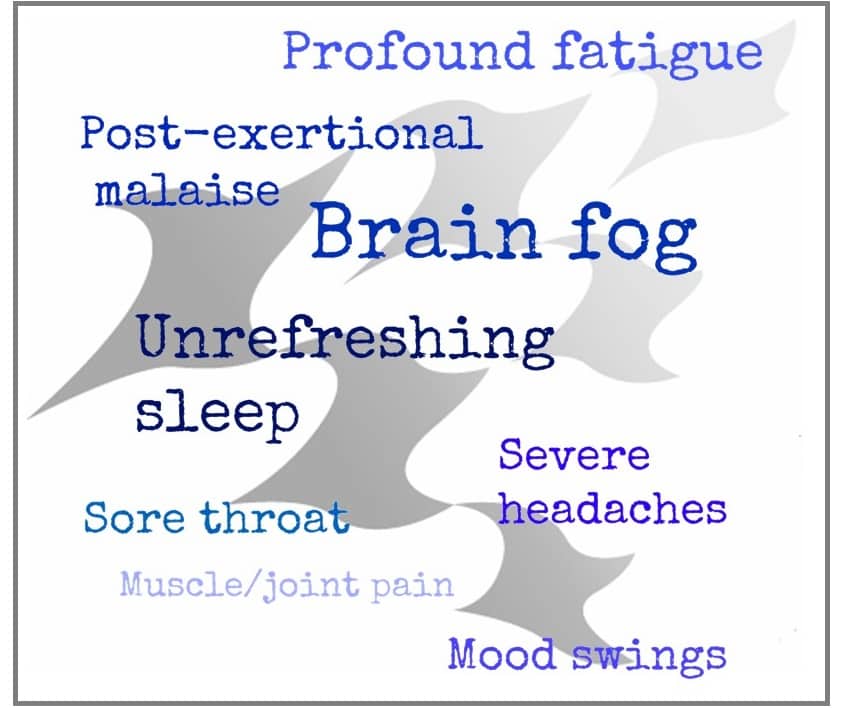
Less than a year later in February 2015 the Institute of Medicine (now called the National Academy of Medicine) published proposed clinical diagnostic criteria for ME/CFS in a report titled, “Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining and Illness”. This was a landmark publication! The report described three core symptoms of ME/CFS as impaired day-to-day function, post-exertional malaise, and unrefreshing sleep as universally present in ME/CFS patients. In addition, either cognitive impairment or orthostatic intolerance must be present as a fourth core symptom for a diagnosis of ME/CFS; plus all core symptoms must be frequently present and at least moderately severe. These clear and concise diagnostic criteria paved the way for health care providers to diagnosis and care for patients.
These core criteria also defined the research roadmap for the Bateman Horne Center.
The BHC mission is to mainstream ME/CFS and FM into medicine. This means giving health care providers the tools they need to diagnose and treat patients. This is an ambitious goal given that we are following Dr. Kweder’s direction by going “back to the core”. But with the universal core symptoms identified, the path forward for BHC is clear. Our objective is to develop tools that can be used to measure the core symptoms making it easier for health care providers to make the diagnosis and to give us ways to measure a change in a core symptom in treatment trials (this is known as an outcome measure).
Here’s how BHC is tackling each of the core symptoms:
- Impaired day-to-day function: measure hours of upright activity (the amount of time over 24 hours a patient’s feet are on the floor)
- Unrefreshing sleep: test affordable wearable devices that can be used at home over long periods of time to get a more realistic picture of disturbed sleep
- Orthostatic intolerance: standardize a 10-minute lean test and measure hemodynamics, heart rate and heart rate variability, skin temperature and galvanic skin response using conventional and wearable devices
- Cognitive impairment: use brain testing software that can assess processing speed and reaction time in minutes to measure brain fog
Standardizing the way the core symptoms of ME/CFS are measured will be a great attractor for the pharmaceutical industry. This will encourage them to look into their pharmacopeia to find drug treatments that can target the core symptoms of ME/CFS. Having the tools to measure the core symptoms is what is needed to get pharma to invest in ME/CFS treatment trials.
BHC is leading the way, working collaboratively with many others to put ME/CFS and FM into the medical mainstream. But we can’t do it alone; you play an important role in our progress.
Join us if you can… wear wild socks, maintain hope, speak up, stand out, volunteer, take action and donate.

 Lucinda Bateman, MD, is a renowned clinician, researcher, and educator. Her Johns Hopkins University Medical School training instilled an approach to care that she has employed throughout her career - the patient comes first and the unknown or unexplained does not equate to a lack of proper and compassionate care. Since starting her own practice in 2000, she has served on six boards or committees, been the principal investigator for 45 studies, authored/coauthored 40 journal articles, served as adjunct instructor and adjunct assistant professor in the University of Utah Departments of Preventative Medicine, Internal Medicine, and Anesthesiology, and lectured around the world.
Lucinda Bateman, MD, is a renowned clinician, researcher, and educator. Her Johns Hopkins University Medical School training instilled an approach to care that she has employed throughout her career - the patient comes first and the unknown or unexplained does not equate to a lack of proper and compassionate care. Since starting her own practice in 2000, she has served on six boards or committees, been the principal investigator for 45 studies, authored/coauthored 40 journal articles, served as adjunct instructor and adjunct assistant professor in the University of Utah Departments of Preventative Medicine, Internal Medicine, and Anesthesiology, and lectured around the world.